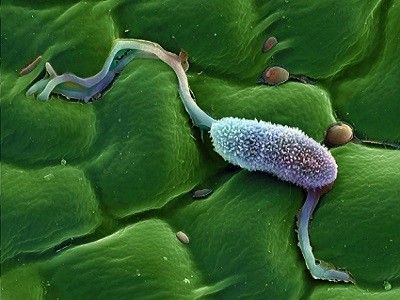
A plant cell containing chloroplasts (darkish inexperienced) — specialised organelles that scientists suppose developed from endosymbionts.Credit score: Dr David Furness, Keele College/Science Picture Library
Scientists wielding a minute hole needle — and a motorcycle pump — have managed to implant micro organism into a bigger cell, making a relationship related to people who sparked the evolution of advanced life.
The feat — described1 in Nature on 2 October — may assist researchers to grasp the origins of pairings that gave rise to specialised organelles referred to as mitochondria and chloroplasts multiple billion years in the past.
Fungi borrowed bacterial gene repeatedly
Endosymbiotic relationships — during which a microbial associate lives harmoniously inside the cells of one other organism — are present in quite a few life types, together with bugs and fungi. Scientists suppose that mitochondria, the organelles which are accountable for cells’ power manufacturing, developed when a bacterium took up residence inside an ancestor of eukaryotic cells. Chloroplasts emerged when an ancestor of vegetation swallowed a photosynthetic microorganism.
Figuring out the elements that fashioned and sustained these couplings is troublesome as a result of they occurred so way back. To get round this downside, a staff led by microbiologist Julia Vorholt, on the Swiss Federal Institute of Know-how in Zurich (ETH Zurich), has spent the previous few years engineering endosymbioses within the laboratory. Their strategy makes use of a 500-1000 nanometre large needle to puncture host cells after which ship bacterial cells one by one.
Sparking symbiosis
Even with this technical wizardry, preliminary pairings tended to fail; for example, as a result of the would-be symbiont divided too quick and killed its host2. The staff’s luck modified once they recreated a pure symbiosis that happens between some strains of a fungal plant pathogen, Rhizopus microsporus, and the bacterium Mycetohabitans rhizoxinica, which produces a toxin that protects the fungus from predation.
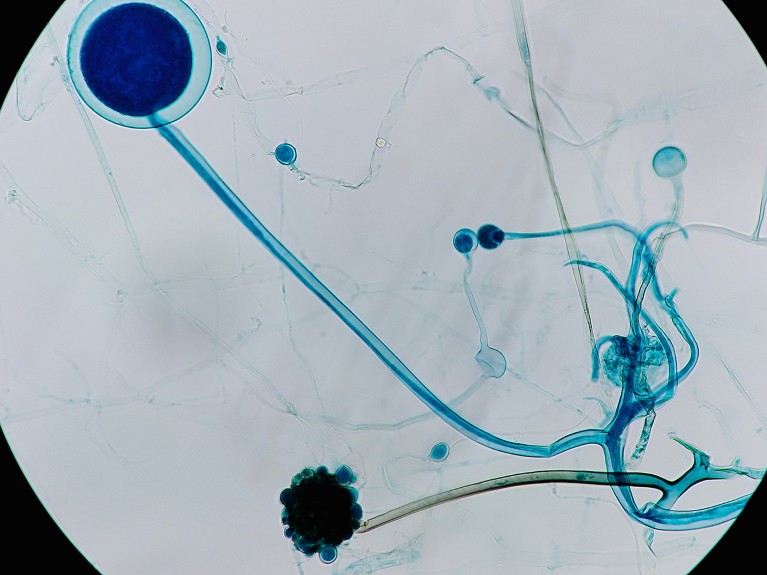
Researchers implanted micro organism into Rhizopus fungi — seen right here underneath a microscope.Credit score: SRMY/Shutterstock
But delivering bacterial cells into the fungi, which have thick cell partitions that preserve a excessive inner strain, was a problem. After piercing the wall with the needle, the researchers used a bicycle pump — and later an air compressor — to take care of sufficient strain to ship the micro organism.
After overcoming the preliminary shock of surgical procedure, the fungi continued their life cycles and produced spores, a fraction of which contained micro organism. When these spores germinated, micro organism had been additionally current within the cells of the subsequent technology of fungi. This confirmed that the brand new endosymbiosis might be handed onto offspring — a key discovering.
Vanishing micro organism
However the germination success of the bacteria-containing spores was low. In a blended inhabitants of spores (some with micro organism and a few with out), these with micro organism vanished after two generations. To see whether or not relations might be improved, the researchers used a fluorescent cell sorter to pick out spores containing micro organism — which had been labelled with a glowing protein — and propagated solely these spores in future rounds of copy. By ten generations, the bacteria-containing spores germinated almost as effectively as these with out micro organism.
The premise of this adaptation isn’t clear. Genome sequencing recognized a handful of mutations related to improved germination success within the fungus — which was a pressure of R. microsporus not recognized to hold endosymbionts naturally — and located no adjustments within the micro organism.
The road that germinated most effectively tended to restrict the variety of micro organism in every spore, says examine co-author Gabriel Giger, a microbiologist at ETH Zurich. “There are methods for these two companions to make a greater, simpler dwelling with one another. That’s one thing that’s actually necessary for us to grasp.”
Fungal immune system
Researchers don’t know a lot in regards to the genetics of R. microsporus. However Thomas Richards, an evolutionary biologist on the College of Oxford, UK, wonders whether or not a fungal immune system is stopping symbiosis — and whether or not mutations to this technique might be easing relations. “I’m an enormous fan of this work,” he provides.
Eva Nowack, a microbiologist at Heinrich Heine College Düsseldorf in Germany, was shocked at how rapidly diversifications to symbiotic life appeared to evolve. Sooner or later, she would like to see what occurs after even longer time durations; for instance, greater than 1,000 generations.
Engineering such symbioses may result in the event of novel organisms with helpful traits, comparable to the flexibility to eat carbon dioxide or atmospheric nitrogen, says Vorholt. “That’s the thought: to herald new traits that an organism doesn’t have, and that will be troublesome to implement in any other case.”