Alena Khmelinskaia desires designing bespoke proteins to be so simple as ordering a meal. Image a merchandising machine, she says, which any researcher may use to specify their desired protein’s operate, measurement, location, companions and different traits. “Ideally, you’ll get the right design that may accomplish all this stuff collectively,” says Khmelinskaia, a biophysical chemist at Ludwig Maximilian College in Munich, Germany.
For the second, that’s only a dream. However advances in computational protein design and machine studying are bringing it nearer to actuality than ever.
Till just a few years in the past, researchers altered proteins by cloning them into micro organism or yeast, and coaxing the microorganisms to mutate till they produced the specified product. Scientists may additionally design a protein manually by intentionally altering its amino-acid sequence, however that’s a laborious course of that might trigger it to fold incorrectly or stop the cell from producing it in any respect.
AI has dreamt up a blizzard of latest proteins. Do any of them truly work?
Machine-learning algorithms have modified the sport totally. Researchers can generate new protein buildings on their laptops utilizing instruments pushed by synthetic intelligence (AI), comparable to RFdiffusion and Chroma, which have been skilled on lots of of 1000’s of buildings within the Protein Information Financial institution (PDB). They’ll establish a sequence to match that construction utilizing algorithms comparable to ProteinMPNN. RoseTTAFold and AlphaFold, which calculate buildings from a sequence, can predict whether or not the brand new protein is probably going fold appropriately. Solely then do researchers have to synthesize the bodily protein and check whether or not it really works as predicted.
In lots of circumstances, it does. “As soon as individuals see the experimental information, they get that this factor can work,” Khmelinskaia says of AI protein design. “There’s pleasure for what is feasible.” This yr’s Nobel chemistry prize committee agrees: AlphaFold and different packages that predict or design protein buildings received their builders the 2024 prize. “That we are able to now predict protein buildings and design our personal proteins confers the best profit to humankind,” the announcement learn.
Nonetheless, the best advantages may very well be but to return. Nature spoke to specialists concerning the greatest challenges going through protein design and what it should take to beat them. Right here’s what they mentioned.
Constructing dependable binders
One of many early challenges for protein designers was to foretell how proteins bind to 1 one other — a serious aim for the pharmaceutical business, as a result of ‘binders’ for a given protein may function medicine that activate or inhibit illness pathways. Generative AI packages comparable to RFdiffusion and AlphaProteo have made this activity easy, says David Baker, a pioneer of computational protein design and 2024 Nobel chemistry laureate on the College of Washington in Seattle, whose staff developed RFdiffusion and different protein-design instruments. “If you wish to goal some most cancers protein, for instance, and also you’d like a binder to it, the strategies we’ve developed will typically offer you an answer to that downside,” he says.
Some proteins, such because the transmembrane molecules that stud the surfaces of immune cells, stay powerful to crack. However for many proteins, generative AI software program can generate binders that wrap exactly round their goal, like a hand. As an example, in 2023, Baker and his colleagues used RFdiffusion to create sensor proteins that gentle up after they connect to particular peptide hormones1.
Chemistry Nobel goes to builders of AlphaFold AI that predicts protein buildings
Protein–protein binding algorithms have been profitable as a result of their language is easy: all pure proteins are made from the identical 20 amino acids. And with lots of of 1000’s of buildings and protein–protein interactions obtainable within the PDB, “that’s type of like a really perfect case for machine studying”, says laptop scientist John Ingraham at Generate Biomedicines, an organization in Somerville, Massachusetts, that makes use of AI to design therapeutics. Groups comparable to his have been utilizing AI instruments to design giant libraries of easy binding proteins, within the hope of making use of them to analysis issues.
However binders change into much less dependable the less information the AI has to coach on, as is the case for proteins meant to bind to medicine and different small molecules. Many pharmaceutical firms have their very own databases of small-molecule buildings and the way they work together with proteins, however these are carefully held secrets and techniques. The general public information that exist aren’t at all times effectively annotated, and the buildings which might be obtainable are likely to signify just some molecular lessons, says Jue Wang, a computational biologist at Google DeepMind in London. “With a mannequin skilled on that, you won’t essentially be taught good normal guidelines about chemistry,” he says.
Earlier this yr, DeepMind launched AlphaFold3, the software program’s newest iteration, which predicts how binding to small molecules impacts a protein’s form. “For the interactions of proteins with different molecule varieties, we see at the least a 50% enchancment in contrast with current prediction strategies, and for some essential classes of interplay we have now doubled prediction accuracy,” the corporate says.
However the problem isn’t fully solved, Baker says. As an example, simply because one thing binds effectively doesn’t imply it should work as meant. A binder protein can activate its goal or block it, however packages comparable to AlphaFold can’t essentially inform the distinction, Khmelinskaia says. (Some algorithms do incorporate operate, she notes, together with ESM3. Developed by an organization known as EvolutionaryScale in New York Metropolis, that software program was skilled on 2.7 billion protein sequences, buildings and capabilities.)

Alena Khmelinskaia is creating hole nanoparticles with the assistance of machine studying.Credit score: Bhoomika Basu Mallik
Generative AI programs produce other limitations, together with an inclination to ‘hallucinate’ protein buildings that can’t exist in nature. The AI is “at all times making an attempt to please”, says Mohammed AlQuraishi, a computational biologist at Columbia College in New York Metropolis. “It by no means, ever says, ‘no, this isn’t doable’.”
A greater understanding of biophysics would possibly assist, Ingraham says, however so would extra and higher information on how proteins bind to molecules. His firm is attacking the issue by brute pressure, utilizing as a lot information on protein interactions and capabilities as potential and mixing it with high-throughput information on designs generated by their mannequin. “We’re looking for normal options,” he says, “then simply leverage as a lot protein data as we are able to.”
New catalysts
Scientists have excessive hopes that computational instruments will result in enzymes with totally new capabilities: catalysts that may scrub carbon dioxide from the ambiance, as an example, or enzymes that effectively break down environmental plastics. The logical place to start out is with pure enzymes that carry out related capabilities. An enzyme that breaks hydrogen–silicon bonds, as an example, would possibly kind the scaffold for a synthetic enzyme that breaks carbon–silicon bonds.
However related protein shapes don’t essentially equate to related capabilities, and enzymes that look nothing alike can perform an identical duties. Understanding these connections — and how one can recreate capabilities — is a serious problem in protein design, AlQuraishi says. “We don’t converse operate, we converse construction.”
Furthermore, pure enzymes aren’t essentially supreme beginning factors for a brand new meant exercise. Debora Marks, a programs biologist at Harvard Medical Faculty in Boston, Massachusetts, likens repurposing enzymes to constructing a contemporary highway system atop a metropolis’s current, antiquated format. “In the event you may begin once more, you wouldn’t essentially do it like that,” she says.
That mentioned, the biophysics of pure enzymes can inform de novo designs, Marks says: “Nature has completed billions of evolutionary experiments for you.” Sometimes, researchers decide which elements of an enzyme are essential by analysing how related they’re throughout species. Evolutionarily conserved sequences typically have related buildings, whereas dissimilar ones would possibly simply be junk that slows an enzyme down.
What’s subsequent for AlphaFold and the AI protein-folding revolution
But it surely’s not at all times instantly obvious which elements are essential, Ingraham says. A seemingly ineffective amino-acid chain on the facet of an enzyme, as an example, would possibly have an effect on how tightly a protein can bind to different molecules or its skill to flip between conformational states.
Some researchers are creating strategies for locating these helpful elements. In an August preprint, Baker and his colleagues used RFdiffusion to create a set of enzymes generally known as hydrolases, which use water to interrupt chemical bonds by a multistep course of2. Utilizing machine studying, the researchers analysed which elements, or motifs, of the enzymes have been lively at every step. They then copied these motifs and requested RFdiffusion to construct totally new proteins round them. When the researchers examined 20 of the designs, they discovered that two of them have been capable of hydrolyse their substrates in a brand new means. “That had been a aim for a very long time, and that’s been solved,” Wang says.
Nonetheless, shifting lively websites into new protein environments may be tough, warns Martin Steinegger, a computational biologist at Seoul Nationwide College. With out the remainder of its protein to stabilize the construction or carry out capabilities that researchers haven’t but recognized, an remoted motif would possibly bind to its goal and by no means let go. Proteins, Steinegger explains, aren’t static objects, however dynamic. “Each time dynamics is available in, we’re simply not likely nice in modelling this.”
Conformational modifications
Proteins typically don’t have only one form; they open, shut, twist and flex. These conformations change relying on components comparable to temperature, pH, the chemical setting, and whether or not they’re certain to different molecules.
But, when researchers try to unravel the construction of a protein experimentally, they typically find yourself seeing solely probably the most secure conformation, which isn’t essentially the shape the protein takes when it’s lively. “We take these snapshots of them, however they’re wiggly,” says Kevin Yang, a machine-learning scientist at Microsoft Analysis in Cambridge, Massachusetts. To actually perceive how a protein works, he says, researchers have to know the entire vary of its potential actions and conformations — different kinds that aren’t essentially catalogued within the PDB.
Calculating all of the methods through which proteins would possibly transfer is astronomically tough, even for a supercomputer. A protein with 100 amino acids — small by protein requirements — may assume at the least 3100 potential conformations, says Tanja Kortemme, a bioengineer on the College of California, San Francisco. “Our understanding of physics is fairly good, however incorporating that is restricted by the variety of prospects we have to compute.”

Protein-design pioneer David Baker on the day it was introduced that he would share within the 2024 chemistry Nobel prize.Credit score: Zach Garcia / UW Drugs
Machine studying will help to slim them down, and Microsoft and different firms are creating methods to hurry up the calculations wanted to discover a protein’s conformation. However AI fashions are restricted by an absence of fine coaching information, Wang says: “Floor fact truly typically doesn’t exist, so how are you aware you’ve even gotten the proper reply?”
Kortemme says the sphere is chipping away at this downside by designing giant libraries of proteins — each pure and artificial — and mutating them to disclose their dynamics. As an example, she, Baker and others are engaged on proteins that may be manually switched between two conformations by including sure binding companions3. Such designer proteins couldn’t solely assist to coach AI fashions but additionally function constructing blocks for more-complex molecular machines, comparable to enzymes that convert chemical power to mechanical power to do mobile work.
Different groups have developed algorithms (comparable to AF-Cluster) that inject a level of randomness into their predictions to discover different conformations. However whether or not these approaches can be relevant throughout protein lessons stays unclear, Steinegger says.
Complicated creations
Enzymes aren’t the one protein class that researchers care about. New proteins may additionally show helpful as constructing blocks, as an example by self-assembling into buildings that carry cargo into cells, generate bodily pressure, or unfold misfolded proteins in issues comparable to Alzheimer’s.
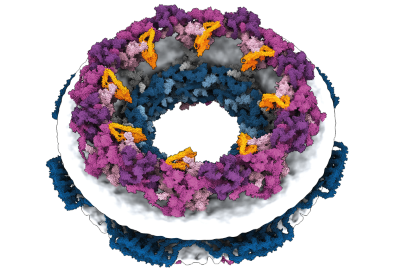
Computational design of those advanced buildings is already making an influence. In 2022 and 2023, respectively, South Korea and the UK accepted emergency use of a COVID-19 vaccine that was the primary medical product created from computationally designed proteins. Generally known as SKYCovione, the vaccine is a nanoparticle with two protein parts that spark an immune response towards the spike protein of the virus SARS-CoV-2. In scientific trials, SKYCovione generated 3 times the extent of antibodies as did a industrial vaccine, and its success, Khmelinskaia says, reveals that computational protein design is prepared for the actual world. “Now it’s actually potential to start out focusing on plenty of fascinating pathways that beforehand have been not likely potential,” she says.
Synthetic intelligence powers protein-folding predictions
Khmelinskaia’s laboratory is utilizing machine-learning algorithms to develop hole nanoparticles that might, amongst different issues, carry medicine or toxins into cells or sequester undesirable molecules. That requires understanding the designed proteins’ conformational dynamics, she says, in that the particle and its payload want to have the ability to cross by the cell’s membrane after which open (or shut).
However that’s only one operate. With a extra advanced construction such because the bacterial flagellum, machine studying can solely achieve this a lot — there simply aren’t sufficient well-understood examples to work from. “If we had 100,000 or one million totally different molecular machines, perhaps we may prepare a generative AI methodology to generate machines from scratch, however there aren’t,” Baker says.
That implies that human researchers want to consider the parts that make up a molecular machine — a motor, as an example, or a protein that ‘walks’ alongside one other protein — and use design instruments to create these constructing blocks one after the other. Such parts would possibly embrace molecular switches, wheels and axles, or ‘logic gate’ programs that solely operate underneath sure circumstances. “You don’t have to reinvent the wheel each time you make a fancy machine,” explains Kortemme. Her lab is designing cell-signalling molecules that may very well be included into artificial signal-transduction cascades.
And it’s within the intelligent recombination of those elements that human ingenuity will come to the fore, Wang says. “We’re beginning to create the screws and bolts and levers and pulleys of proteins,” he says. “However what are you going to make use of that pulley for? That’s probably the most fascinating and probably the most difficult facet.”
Studying from errors
Khmelinskaia’s vending-machine imaginative and prescient however, even one of the best prediction algorithms are a way from creating an correct protein in a single take. “It was once that 99.99% of the time, it doesn’t work,” AlQuraishi says. “Now it’s extra prefer it solely fails 99% of the time.”
NatureTech hub
That’s partly an issue of logistics, Steinegger says. Computational researchers can run their algorithms time and again till they discover one thing that appears like it should work, and algorithm-design groups comparable to his personal “have new improvements about each three or 4 months”. Verifying the designed proteins in a organic system, Steinegger estimates, would possibly take two years, by which level the software program has already moved on.
This mismatch implies that algorithms hardly ever get the prospect to be taught from their errors. Researchers have a tendency to not publish adverse outcomes, even when these failures yielded probably helpful data comparable to a protein’s mobile toxicity or stability underneath sure circumstances. Barring radical modifications in scientific funding fashions to incentivize such disclosures, researchers should get artistic. “It’s extraordinarily difficult to construct a staff that really can cowl all these sides without delay,” Khmelinskaia explains, referring to the bench and computational sides of protein-design analysis. So, collaboration is a should.
“We’re type of at this stage the place the pc assets and the information are each prepared, and that’s why it’s change into such a preferred discipline,” Yang says. “The extra individuals work collectively, the sooner they progress.”