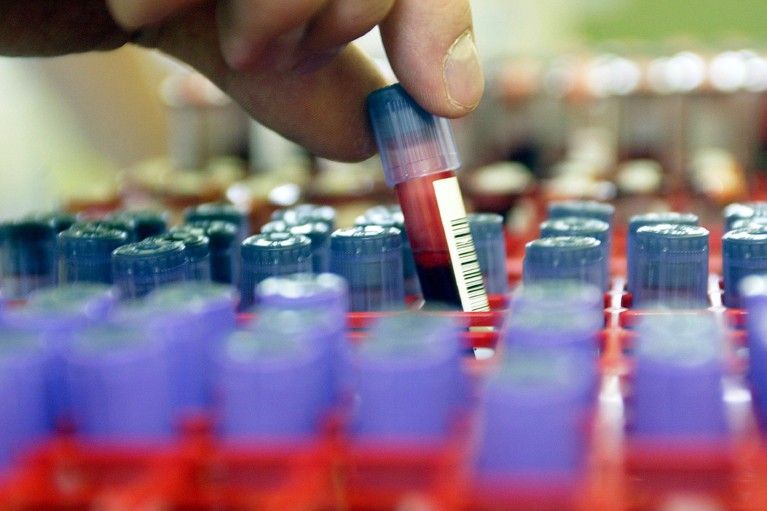
Scientists have developed a blood check that makes use of biomarkers to assist diagnose bipolar dysfunction.Credit score: John Thys/Reporters/Science Picture Library
A primary-of-its-kind blood check that makes use of biomarkers to tell apart bipolar dysfunction from melancholy may slash the time it takes to get an correct analysis from years to weeks, in accordance with the corporate that developed the check — however some scientists have raised considerations about its validity.
The check makes use of biomarkers associated to RNA enhancing to diagnose the situation and has been obtainable in France since March and in Italy since October 2023, having been granted regulatory approval in each international locations.
Blood check makes use of ‘protein clock’ to foretell threat of Alzheimer’s and different ailments
Nevertheless, some researchers are involved in regards to the small measurement of the trials the check is predicated on and the dearth of impartial verification of the research. The check’s developer, the French start-up Alcediag in Montpellier, says that its trials are legitimate and reproducible.
The row highlights a wider dialogue in regards to the potential of biomarkers — organic traits that may point out a selected medical state — to allow earlier analysis and extra personalised therapies for psychiatric issues.
“There’s a function for investigating biomarkers,” says Suresh Sundram, a psychiatrist at Monash College in Melbourne, Australia. “However it’s a very fraught space.”
Gradual analysis
Bipolar dysfunction, a spectrum of situations characterised by temper swings that alternate between mania and melancholy, is tough to diagnose. About 40 million folks globally reside with the sickness, and the diagnostic course of, which frequently entails a number of periods with a psychiatrist, takes a mean of seven to 10 years, throughout which period individuals are usually misdiagnosed with situations comparable to melancholy and given inappropriate or ineffective therapies.
Alcediag hopes to change this grim panorama. The corporate says its €900 (US$980) blood check, EDIT-B, will help to tell apart bipolar dysfunction from melancholy utilizing biomarkers. EDIT-B differentiates between the 2 by measuring refined variations in RNA enhancing — a regulatory course of that alters numerous mobile mechanisms, together with the expression of genes, which in flip impacts neurological functioning.
Discovered: a brain-wiring sample linked to melancholy
A number of research have instructed that variations in RNA enhancing may play an element in autoimmune ailments and most cancers, in addition to in psychiatric situations. In preliminary analysis, scientists at Alcediag recognized distinct patterns of RNA enhancing affecting eight genes that appear to vary between wholesome folks and people experiencing melancholy. Among the many depressed sufferers, six of those genes additionally present variations that distinguish folks with melancholy from these with bipolar dysfunction. These variations produce a singular mixture — or signature — of biomarkers that the corporate says it found utilizing a man-made intelligence (AI) algorithm it developed.
“Now we have a signature for these with melancholy, a signature for the controls, and … one for bipolar,” says Dinah Weissmann, co-founder and chief scientific officer of Alcediag. In a 2022 examine involving 410 contributors, the algorithm distinguished between the 160 folks with melancholy and the 95 people with bipolar dysfunction with excessive accuracy1.
About 80 folks have used EDIT-B since its commercialization in France and Italy, says Weissmann, and suggestions has been optimistic to date. She cites the anecdotal report of an individual who, after receiving a optimistic check consequence, mentioned they modified to more practical treatment. “The affected person wrote to their physician: ‘It’s nice, I’m again on my toes. I’m dwelling usually once more,’” says Weissmann.
Potential dangers
For many individuals with bipolar dysfunction, a quicker, extra exact analysis would enable them to entry “the appropriate treatment, on the proper time”, says Marion Leboyer, a psychiatrist and government director of the FondaMental Basis, a analysis organisation primarily based close to Paris.
However, if a blood check provides an incorrect consequence, there’s a threat that issues could possibly be misdiagnosed or neglected, says Boris Chaumette, a psychiatrist on the French Nationwide Institute of Well being and Medical Analysis in Paris.
There isn’t a indication that an EDIT-B consequence has led to an incorrect analysis. However Chaumette and others are involved about a few of the methodology of research used to reveal the EDIT-B check’s effectiveness. He factors to “inherent limits” to the 2022 examine with 410 contributors. “You’re taking an information set with many variables and never many sufferers, and also you ask an algorithm to categorise folks. It is going to inevitably discover issues that classify them, it is going to inevitably determine commonalities,” he says. “In reality, what you observe could possibly be the impact of therapies.”
Easy blood check detects eight totally different sorts of most cancers
He provides that, other than the management group, each individual concerned in Alcediag’s research was taking treatment (as is commonly the case with psychiatric analysis). These medication may have an effect on the degrees of some biomarkers, says Sundram, “so the algorithm may choose up the impact of the drugs”.
Weissmann says that the examine contributors with bipolar dysfunction and people with melancholy had been taking a variety of various drugs, so if the algorithm was distinguishing folks on the premise of their therapies, it could have categorised them in accordance with therapeutic lessons. Secure sufferers — those that had a analysis however weren’t experiencing signs on the time of the examine — additionally had a special biomarker signature from the management group, she provides, indicating that their treatment suppressed signs with out affecting markers of the underlying sickness. She says that Alcediag’s research have concerned a whole bunch of contributors, and that the corporate is conducting a brand new medical trial with 436 sufferers; they count on to publish the outcomes subsequent yr.
Replication questions
Sure points of Alcediag’s research make it tough for impartial researchers to confirm the work, says Chaumette. The algorithm and its underlying code haven’t been shared, and follow-up research run after the preliminary 2022 trial have used barely totally different variations of the check. In a examine the agency revealed this yr, for instance, it had eliminated one biomarker from the preliminary mixture and added three new ones2.
This problem led the French Nationwide Authority for Well being (HAS), an impartial physique that assesses well being merchandise, to reject Alcediag’s software for folks taking the check to be reimbursed by the French well being authorities. “There have been variations in efficiency among the many three variations, and we lacked the rationale to clarify why this model was chosen and never one other,” says Cédric Carbonneil, who heads the HAS division liable for assessing new medical units and procedures. It isn’t uncommon for firms at early levels of growth to initially have their functions denied, and the HAS expects Alcediag to reapply, he provides. Alcediag says that it made adjustments to the biomarkers to enhance the check’s efficiency, and that it hasn’t shared the algorithm for business causes.
Chaumette says he would have preferred to see bigger research backing up the check earlier than it was rolled out to sufferers, and he hopes Alcediag will make its know-how obtainable to impartial teams to permit them to duplicate the findings. “Should you commercialize it quickly and patent the whole lot with out sharing something, then it turns into opaque.”