From roundworms to people, signalling by the Notch protein-receptor household drives embryonic improvement, mobile differentiation and tissue homeostasis. It’s additionally completely important for the transformation of immature human immune cells into T cells — mobile warriors that concentrate on viruses and tumours.
But the activation of Notch signalling has been onerous to imitate within the laboratory, making it near not possible to use within the clinic and hampering efforts to generate T-cell therapies. However that may very well be altering.
In July, attendees at a convention in Lewiston, Maine, devoted to Notch signalling had been handled to not one, however two new instruments to activate the pathway. Unbeknown to one another, two analysis teams had been working in parallel to develop what they unveiled on the convention.
“It was a turning level,” says Juan Carlos Zúñiga-Pflücker, a developmental immunologist on the College of Toronto in Canada, whose lab has pioneered many Notch activation strategies. For him, the bulletins represented a “form of a re-emergence” of this work within the Notch subject.
Bother with 2D
Most receptors endure some form of conformational change after binding to their ligands, resulting in a series of occasions that alters the cell’s behaviour. However ligand binding alone isn’t sufficient to activate Notch.
As soon as the receptor binds to its ligand (which is tethered to a different cell), the ligand-expressing cell reels within the ligand-receptor complicated. Like tugging at a unfastened thread in a sweater, the ensuing pulling pressure unravels a part of the Notch receptor, exposing it to enzymes that launch the receptor’s intracellular area and permit it to journey to the nucleus to affect gene expression.
Stealthy stem cells to deal with illness
“The true technological problem is developing with a drug that may mimic that pulling impact,” says Vincent Luca, a protein engineer on the Moffitt Most cancers Middle in Tampa, Florida.
Present methods require the co-culture of cells expressing the Notch receptor with cells that specific the ligand, or fixing the ligand onto plates or beads. However these ‘2D’ strategies can’t be used in vivo, says Luca.
They’re additionally a bottleneck for large-scale T-cell manufacturing, says George Daley, a haematologist and stem-cell biologist at Harvard Medical Faculty in Boston, Massachusetts, as a result of solely the cells at that ligand-presenting interface will be activated. For years, Daley’s lab has struggled with the constraints of 2D strategies whereas making an attempt to coax pluripotent stem cells into T cells.
In 2021, Daley was approached by Rubul Mout, then a postdoctoral researcher on the College of Washington in Seattle within the lab of David Baker, who gained a share of the 2024 Nobel Prize in Chemistry for his work in computer-aided protein design. Mout wished to use these design strategies to Daley’s stem-cell-based techniques.
Daley was “tremendously excited” on the concept, and earlier than Mout even formally joined his lab, they mentioned engineering a soluble Notch agonist — one that would current a Notch ligand to cells in suspension moderately than on a flat floor.
Turbocharged CAR-T cells soften tumours in mice — utilizing a trick from most cancers cells
Utilizing the Rosetta protein-design instrument developed in Baker’s lab, Daley, Mout and their colleagues designed potential agonists by attaching a number of copies of the ligand to a protein scaffold. They experimented with designs with as few as two and as many as 120 copies of the ligand, in addition to completely different geometries.
One association labored notably properly: three copies of the ligand radiating out from the scaffold, like spokes. When added to Notch-expressing cells in suspension, this multivalent ligand linked the cells collectively in a microscopic pas de deux, with every growing T cell gently pulling on (and activating) the opposite. In contrast with 2D activation, Notch activation utilizing the soluble agonist in a bioreactor created 5 instances extra T cells per microgram of agonist1, suggesting that this method is perhaps helpful for large-scale T-cell manufacturing.
Boosting Notch
Luca says that his group, against this, was trying to find a “focused, targeted manner of activating Notch”, with a watch to therapeutics. For instance, boosting Notch might rejuvenate exhausted T cells making an attempt to destroy a tumour — however this should be completed with out activating the tumour cells, which additionally specific Notch.
Luca’s lab generated SNAGs, artificial Notch agonists (see ‘Mimicking notch’), by fusing a gene for a Notch agonist to at least one that encodes an antibody fragment concentrating on most cancers cells. The outcome encodes a bispecific protein that kinds what Luca calls a “little molecular bridge” between a Notch-expressing cell and the cancer-antigen-expressing cell. That binding occasion mechanically prompts the receptor in a fashion akin to pure Notch signalling2.
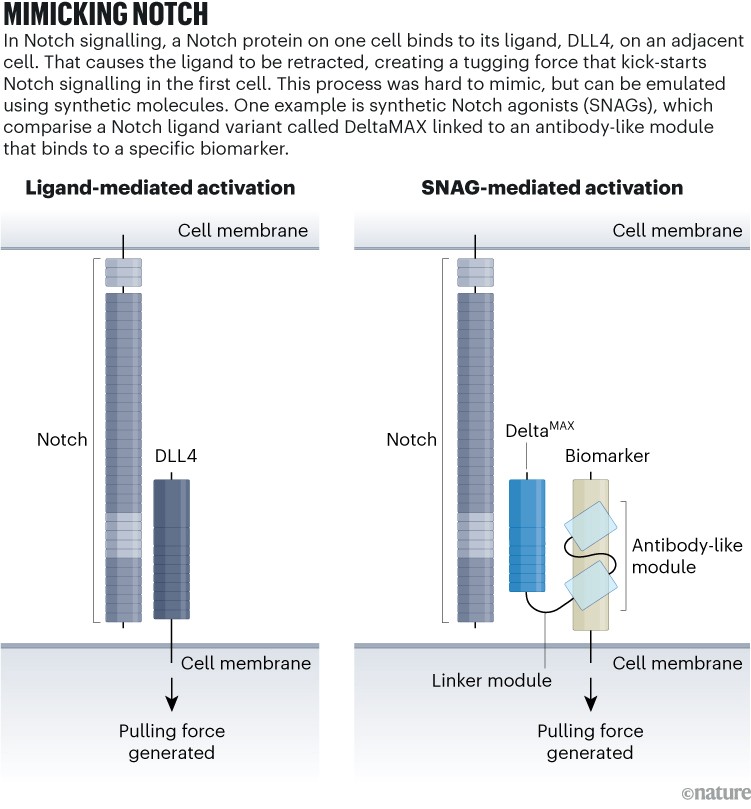
Supply: Tailored from ref. 2
A really completely different soluble Notch agonist was unveiled in January. Biophysicist Björn Högberg and his group on the Karolinska Institute in Stockholm research the minuscule, tactile interactions between proteins throughout cell signalling, which Högberg likens to “a Braille system” that the cells use to speak to at least one one other.
Working with Luca, the group created a hybrid DNA-protein agonist by attaching a number of copies of the Notch ligand alongside a chunk of DNA that had been folded, origami-style, right into a rod. To their shock, the agonist activated Notch with out producing any measurable pulling pressure3.
The researchers recommend that their origami agonist is perhaps triggering an alternate mechanism of activation, by which long-term ligand binding is enough to trigger receptor unravelling, albeit at a a lot slower tempo. However they’re nonetheless making an attempt to know how this would possibly work.
Extremely mutated cancers reply higher to immune remedy
Reactions from the group about the potential for a pulling-independent Notch activation mechanism have been blended, Högberg says: “I feel a few of them had been actually intrigued and actually, actually wished to know extra, and a few of them had been like, ‘I don’t assume that is something.’”
For his half, Zúñiga-Pflücker is sceptical that the origami ligand works with none form of pulling pressure. However “the work is de facto properly completed”, he says, including that “all three papers are fairly thrilling in numerous methods”.
Even just some years in the past, says Mout, de novo protein design wasn’t superior sufficient to deal with significant biology. However the subject is quickly evolving, he says, and actual issues are being solved. “Every part is coming collectively now,” he says.